1. You must satisfactorily complete the assigned laboratory experiments. (Missing 3 or more will constitute an F or W for the course)
2. You must participate in weekly recitations (missing 3 or more may constitute an F for the course)
3. You must complete the weekly quizzes and hour tests, as assigned.
4. You must achieve a passing grade on a comprehensive final examination.
5. You must complete a minimum of six hours of work on chemistry at home each week.
6. You must demonstrate your level of performance (see page 3 for "grading") by mastering a large part of the material covered by lectures, films, homework, laboratory work and the textbooks as detailed in the specific course objectives that follow.
7. With a C or better in CHE 101 you will be expected to know the material in that course and you can expect test questions from that course.
Unit II: Chemical Kinetics: 1. Define the following terms (using examples where appropriate) especially in terms of a "reaction profile curve": a. DH reaction e. Exothermicb. Activation energy, Ea f. Endothermicc. Reaction coordinate g. Activated complexd. Transition state h. Reaction coordinates 2. Define the rate of a chemical reaction. 3. Given any two of the following: Eaf, Ear, DH; calculate the third, and locate them on a "reaction profile" or Arrhenius diagram. 4. Account for the rate or reaction in gas phase reactions in terms of collision of molecules. 5. Explain what is meant by effective collisions and why so few collisions result in product molecules being formed. 6. Name all six factors: Nature of reactants, state of subdivision, temperature, catalysis, concentration, and pressure (gas reactions) upon which the rate of reaction depends, and explain where they appear in the rate law. 7. Explain how each of the above factors affects the rate of reaction including your discussion of the "reaction profile curve", and of the "molecules eye-view" (collision theory). 8. Differentiate among a homogeneous catalyst, a heterogeneous catalyst, and an inhibitor. 9. With respect to rate laws, define the terms (using examples where appropriate): a. Rate e. Reaction mechanismb. Rate constant f. Elementary process or stepc. Order of the reaction g. Rate determining stepd. Molecularity 10. For a given reaction such as: A + 3 B ssssd 2 C a. Describe the rate of reaction in terms of the disappearance of A or of B or the formation of C.b. Quantitatively correlate the rate of disappearance of A to that of B as well as to the rate of formation of C.c. Write a general rate law for the reaction.11. Given the measured initial rates of a reaction: k a A + b B sssssd products where the initial concentration of each reactant is varied over a sufficient number of trials, determine the rate law: Rate = k [A]x [B]y including x, y, and k values and
the order of the reaction. 12. Once a rate law is known, determine an initial rate given any set of initial concentrations. 13. Write the rate law for an elementary process. 14. Describe reaction mechanisms as a sum of elementary processes. 15. Given a number of steps in a reaction mechanism, and the rate constant of each step, identify the rate-determining step with the slowest step in the entire mechanism and determine the rate law for the overall reaction. 16. Given the mechanism and rate law, determine which step is the rate determining step. 17. Explain the sequence in a chain reaction. Unit III: A. Chemical Equilibrium: 1. Define and explain the law of mass action, equilibrium, and equilibrium constant. 2. Write the mass action expression for any reaction given a balanced chemical equation. 3. For a reversible chemical reaction: a A + b B qwe c C + d D derive the equilibrium constant expression: [C]c [D]d Kc = ¾¾¾¾¾¾ [A]a [B]b by utilizing the dynamic equilibrium concept, (i.e., rate forward = rate reverse at equilibrium). 4. Given the concentration of all the products and the reactants involved in a reversible reaction, determine the numerical value of the equilibrium constant for that reaction. 5. Know that the equilibrium constant is a constant at a given temperature. 6. State LeChatelier's principle in your own words and apply it to a given system at equilibrium under the change of one of the following factors: temperature, concentration, pressure or volume (gas reactions only), addition of inert gases, addition of a catalyst; and to predict the direction of shift in the equilibrium position as well as the change (or lack of change) in the value of Kc due to each of the above factors. 7. Differentiate between a homogeneous equilibrium and a heterogeneous equilibrium. 8. In a heterogeneous equilibrium between gaseous and liquid solutions, represent the concentration of each gaseous species by its partial pressure raised to the appropriate power and the concentration of each species in liquid solution by its concentration in moles per liter. 9. In a heterogeneous equilibrium between gaseous and liquids or solids, note that the concentrations of the liquids or solids is constant and write the appropriate law of mass action. 10. Where it applies, define the equilibrium constant in terms of partial pressures only (Kp) and note that, while Kp is still a constant, it has a different value than Kc. 11. Calculate Kc from Kp and vice versa. 12. Differentiate between concentrations and activities. 13. Given the equilibrium constant, Kc, numerically, and the initial concentrations and/or partial pressures of all reactants and products involved, calculate the concentrations and/or partial pressures of all species involved at equilibrium. 14. Given initial concentrations and one equilibrium concentration, calculate Kc and the other equilibrium concentrations, and vice versa. 15. Convert natural logarithm into logarithm to the base 10: DGo = - 2.303 RT log K
16. State the relationship between the Gibbs free energy and the mass action expression, Q: DG = DGo + RT In Q [C]c [D]d Q = ¾¾¾¾¾¾ [A]a [B]b for a reaction such as: a A + b B qwe c C + d D and derive the relationship between the standard free energy change of the reaction and the equilibrium constant: DGo = -RT ln Kby using the thermodynamic criteria for an equilibrium: DG = O and Q = K 17. Given the value of DGo for a given reaction at a given temperature you shall be able to calculate K for the reaction and vice versa. 18. Understand the meaning, use, and conditions of the relationship: K2 DH (T2 - T1) Log ¾¾ = ¾¾¾¾¾¾¾¾ K1 2.303 R T1T2 19. Assuming that DHo and DSo are independent of temperature and given the equilibrium constant at a temperature T1, and the above equation, calculate the equilibrium constant at a new temperature T2. Unit III: B. Spectrophotometry: 1. Define the terms percent transmission (%T) and absorbance (A) and calculate one from the other. 2. Standardize and take data (%T or A) from a Spectronic 20 spectrophotometer. 3. Plot and explain the absorbance versus wavelength graph for a substance and determine the maximum absorbance. 4. State the Beer-Lambert law: A = abc and explain all letters in it. 5. Plot and explain a Beer-Lambert law graph and use it to find the concentration or absorbance of a substance, given one of them. 6. Calculate the absorbtivity constant, the absorbance, or the concentration of the solution from the Beer-Lambert Law given any two of them and the path length of the solution (b). 7. Apply the above to state and explain the equilibrium which forms Fe(SCN)2+, and how to find K for the equilibrium. Unit IV: Electrochemistry: 1. Balance oxidation-reduction (Redox) reactions and name the oxidizing agent and reducing agent. 2. Given a balanced half reaction, find the equivalent weight of either the oxidizing agent or the reducing agent. 3. Given the number of equivalents of an oxidizing agent used in a redox titration at the end point, find the number of equivalents of the reducing agent, and vice versa. 4. Given the volume and the normality of the oxidizing agent, calculate the normality of the reducing agent, if you were given its volume at the end point in a titration, and vice versa. 5. Relate the weight, equivalent weight, and normality in a redox titration and use them in calculations. 6. Define the following terms, using examples where appropriate: a. Oxidation h. Faradayb. Reduction i. Coulombc. Electrolytic cell j. Electroded. Electrolysis k. Cell potentiale. Voltaic or galvanic cell l. Standard potentialf. Cathode m. Electromotive force or emfg. Anode n. Reduction potential 7. Differentiate between a strong electrolyte and a weak electrolyte by their abilities to conduct a direct current of electricity. 8. In cells, distinguish between electrolytic and voltaic (or galvanic cells), metallic and electrolytic conduction, cathode and anode. 9. Predict the electrode reactions (at the anode and cathode) that occur during the electrolysis of molten sodium chloride and other salts, and aqueous sodium chloride (dilute and concentrated); and be able to draw a diagram of the cells. 10. Define Faraday's law of electrolysis. 11. Quantitatively associate the number of Faradays (Coulombs, or Amps) of electricity passing through the cell with the number of equivalents of the element being reduced at the cathode (mostly metallic elements and also the hydrogen gas from an acid solution), or oxidized at the anode, and with calculations in either direction. 12. Given the half reactions, construct and describe a diagram of a galvanic or voltaic cell. 13. Give the diagram, reaction, and purpose of the standard hydrogen electrode. 14. Given a standard hydrogen electrode (or any other half reaction) and an accurate differential voltmeter, measure the standard reduction potential of a cell formed with another half reaction, and calculate the reduction potential of that half reaction. 15. Diagram a complete Voltaic cell consisting of two half cells, label the anode, the cathode, the salt bridge, the direction of electron flow, the direction of the cation flow or the anion flow across the salt bridge, and explain what occurs at the anode and cathode. 16. Given the standard reduction potentials of two standard electrodes, couple them to obtain a positive standard cell potential, and determine the direction in which the reaction will be spontaneous. 17. Write the short-hand notation for an electrochemical cell according to the convention. Example: Zn(s) ½ Zn2+(1M) ½½ Cu2+(1M) ½ Cu(s) Eo = 1.10 v and to construct a diagram of the cell from the notation. 18. Define and use the electromotive series to determine cell potentials, reactions and spontaneity. 19. Predict the effect of concentration changes on the potential of a cell. 20. Give the relationship between Gibbs free energy and the cell potential: DG = - nFE or DGo = - nFEo and use it to calculate the Gibbs free energy or the cell potential.
21. Using the previous relationships and the thermodynamic relationship: DG = DGo + RT 1n Q derive the Nernst equation: E = Eo -
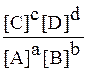 for a reaction 22. Use the Nernst equation to: a. Show that E = Eo when concentrations (or pressures) are the standard state values of 1M (or 1 atm). b. Show that Eo =
for a reaction 22. Use the Nernst equation to: a. Show that E = Eo when concentrations (or pressures) are the standard state values of 1M (or 1 atm). b. Show that Eo = Unit VI: Ionic Equilibria: 1. Define the following terms, using examples where appropriate: a. Ionization constant i. Common ion effectb. pH and pOH j. Complex ionsc. Weak electrolyte k. Instability constantd. Dissociation l. Formation constante. Polyprotic acid m. Hydrolysisf. Indicator n. Solubility product constantg. Buffer o. Equivalence pointh. Hydrolysis constant p. Endpoint 2. Describe the ionization of water and its ionization constant. 3. Calculate the hydrogen ion concentration and the hydroxide ion concentration of pure water. 4. Given one of the following: [H+], [OH-], pH, or pOH of a solution, calculate the other three. 5. Given the concentration of a strong acid or strong base, calculate the pH and pOH of the solution.6. Calculate the pH of weak acids or bases, given their equilibrium concentrations, and vice versa. 7. Given the ionization constant and the initial (total) concentration of a monoprotic weak acid, or a monohydroxy weak base, calculate the hydrogen ion concentration and the hydroxide ion concentration and the concentration of all other species of the solution at equilibrium (you should make the appropriate assumptions). 8. From the information given in (7), calculate the percent ionization in a monoprotic weak acid solution or in a monohydroxy weak base solution. 9. Calculate the equilibrium concentrations of all species present when a polyprotic weak acid dissociates. 10. Given the initial (or total) concentration of a monoprotic weak acid or a monohydroxy weak base and given the pH or the solution at equilibrium, calculate the ionization constant of the weak acid or the weak base, and vice versa. 11. Explain the nature, preparation and use of buffer solutions. 12. Given the initial concentrations of a weak acid and its salt or that of a weak base and its salt (buffers) and the ionization constant(s), deduce the equili-brium conditions with proper assumptions and calculate the resulting pH of the mixture at equilibrium, and after dilution or small additions of acids or bases. 13. Use the common ion effect to calculate equilibrium concentrations or the equilibrium constant for weak acids or weak bases, given initial concentrations of all species. 14. Illustrate the three kinds of hydrolysis: a. Salt of a strong base and a weak acidb. Salt of a weak base and a strong acidc. Salt of a weak base and a weak acid and apply the ideas of hydrolysis to calculate the concentration of all ions and the pH at equilibrium, given initial conditions, or predict the acidic, basic, or neutral nature of salts in water. 15. Standardize a pH meter and correctly measure the pH of a solution with a pH meter. 16. Given the ionization constant of a weak acid or a weak base, and given the weak acid or the weak base and a salt (strong electrolyte) of the acid or of the base and necessary apparatus, prepare a required volume of a buffer solution of a desired pH.17. Given the initial concentration of the titrants and the ionization constants where applicable, predict the end point and the shape of a titration curve of pH against volume of acid or base added to a base or to an acid, respectively, for the following cases: a. Strong acid titrated with a strong base (or the reverse)b. Weak acid titrated with a strong basec. Strong acid titrated with a weak base. 18. Calculate the pH at any point of the addition in (17). 19. Perform any of the titrations in (17) in the laboratory, properly using burettes. 20. Explain the nature of the curve in the titrations of (17) in terms of the vertical rise and the two plateaus and why they are so. 21. Explain an alternate means of preparing the solution that exists at the end point in (17). 22. Select the right indicator according to the range of pH in which the end point of an acid-base titration lies. 23. Calculate the solubility (or concentration of the ions) given the solubility product constant, and vice versa. 24. Use the common ion effect to calculate equilibrium concentrations or the equilibrium constant for slightly soluble salts, given initial concentrations of all species. 25. Given the concentration of a solution of a cation and the concentration of a separate solution of an anion, of a slightly soluble salt, and given its Ksp, mathematically determine if a precipitate will form if given volumes of the two solutions are mixed. 26. Given the information in (25), for the case where a precipitate forms, calculate the number of moles (and grams) of the solid formed, the percent precipitation, and the final concentration of each of the ions remaining in solution. 27. Predict, mathematically, which ion will precipitate when a precipitating agent is added to a solution of two or more ions. 28. Determine the molar solubility of salts in solvents that form complex ions with the solute added. 29. Write instability constant and formation constant expressions from the chemical equation. 30. Relate the instability constant to the formation constant for complex ion formation.Unit VII: Chemistry of the Representative Elements I: The Metals: 1. Distinguish among metals, nonmetals, and metalloids (semi-metals) with respect to chemical properties, physical properties, and positions in the Periodic Table. 2. Write the outer shell electron configuration of any of the representative elements. 3. From the electron configuration of any element, determine which family or group it belongs to and vice versa. 4. Describe the reactions of the representative metals, their oxides, and their hydroxides with water, acids, or bases. 5. Describe the trends in metallic behavior , electronegativity, ionization energy, electron affinity, and atomic radii throughout the periodic table. 6. Deduce, using simple thermodynamics, what type of chemical reaction can be used to produce free metals from their compounds. 7. Illustrate some similarities in chemical behavior of the Group IA, IIA and IIIA elements, especially diagonal relationships, and the relative reactivities within each group. 8. Interpret diagonal relationships in terms of ionic potential. 9. Predict and explain the values of the stable oxidation states for the representative metals, and which will be more stable. 10. Interpret the trends in oxidation states exhibited by the atoms within a group in terms of the relative stabilities of high and low oxidation states. 11. Describe trends in any row or column of the periodic table with respect to: a. Atomic radius f. Metallic propertiesb. Ionic radius properties g. Oxidizing/reducing propertiesc. Ionization potential h. Ionic potentiald. Electron affinity i. Polarization of ionse. Electronegativity j. Hydrolysis 12. Use ionic potential to compare the relative degree of ionic-covalent bonding and physical properties of compounds composed of the representative elements. 13. Discuss the Solvay Process14. Relate equivalents, equivalent weight, weight, normality, and volume in an acid base titration, and use it in calculations.
Unit VIII: Chemistry of the Representative Elements II: The Metalloids and Nonmetals:1. Define the following terms, including examples where appropriate: a. Allotropism e. Disproportionationb. Catenation f. Polymersc. Three center bonds g. Oxoaniond. Amorphous h. Hydride 2. Compare metalloids and nonmetals in terms of the oxidation states displayed and the processes employed in their production. 3. Contrast the methods of preparation of the metalloids with those for the production of the nonmetals. 4. Describe the molecular structure, bonding, geometry and name of the allotropic forms of the pure metalloids and nonmetals. 5. Predict the important oxidation states of the nonmetals and metalloids. 6. Determine the oxidation state of the nonmetals and metalloids in ions and in neutral compounds. 7. Illustrate examples of catenation among nonmetals and metalloids by drawing structural formulas, and which element does it best. 8. Describe the two general methods for the preparation of nonmetals and metalloid hydrides. 9. Relate the ease of preparation and stability of nonmetal and metalloid hydrides to their standard enthalpies and free energies of formation. 10. Compare the relative acidic strength of the hydrides for the elements in both vertical columns and horizontal rows. 11. Write equations for the hydrolysis of nonmetal anions such as sulfide, nitride, phosphide and carbide. 12. Draw the structure of diborane and describe the bonding in this substance and why BH3 is not the simplest stable boron hydride. 13. Write the equation for the reaction of nonmetal oxides with water. 14. Describe three methods of preparation of nonmetal oxides, writing chemical equations for each. 15. Give the formulas of the important nonmetal oxides. 16. Give the structures, hybridization, and resonance forms of NO, NO2, CO, CO2, SO2, SO3 and show the valence bonds ( d and p ) that form. 17. Write equations for the preparation of the compounds in (16). 18. Compare the structures of nonmetal oxides on the bases of bonding preferences exhibited by the non-metals. 19. Compare the structures of P4, P4O6 and P4O10, and give reaction for the preparation of the oxides from phosphorous. 20. Discuss the molecular structure of quartz. 21. Give examples of 3 methods of preparing oxoacids and their anions. 22. Given the structure or the name or the formula for the following oxoacids (and the oxoanions), given one of them: a. HClO f. H2SO4 k. H3PO3 b. HClO2 g. H2S2O3 l. H3PO4 c. HClO3 h. HNO2 m. H2CO3 d. HClO4 i. HNO3 n. H3BO3 e. H2SO3 j. H3PO2 o. H2C2O4 and extend these structures to other members of the same families where appropriate. 23. List the oxidation state for the central element in the oxoacids and oxoanions in (22). 24. Give the bonding, geometries, resonance forms, and hybridization (where appropriate) for the oxoacids and oxoanions in (22). 25. Give the names and structures for the salts which form from the oxoacids in (22). 26. Give the equation for the formation of the oxoacids from the anhydrides, where appropriate (from CO2, N2O3, N2O5, P4O6, P4O10, SO2, SO3). 27. Compare the acidic strengths and oxidizing abilities of the oxoacids of the nonmetals. 28. Predict formulas for the halogen compounds of the nonmetals. Give geometries for these compounds based on the electron repulsion theory and list hybridizations where appropriate. 29. Use electronic structure and relative size of the atoms to determine possibility for existing and relative stability of the nonmetal halogen compounds. 30. Compare and explain the relative reactivities among the halogens and among the noble gases. 31. Explain the valence bond formation in the N2 molecule. 32. Explain why nitrogen is relatively unreactive, when compared to other nonmetals. 33. Define and explain nitrogen fixation and the nitrogen cycle in nature. 34. Discuss the preparation of and bonding in noble gas compounds. Include geometries and hybridization. 35. Indicate the composition of the two most abundant components of the atmosphere. 36. Indicate the six major pollutants of the air. 37. Indicate five sources for these pollutants. Unit IX: The Transition Elements: 1. Describe similarities and differences between A and B groups of the periodic table. 2. Distinguish between representative, transition, and inner transition elements. 3. Explain why Group IIB is sometimes considered a representative group. 4. Compare the properties among the transition elements horizontally as well as vertically including atomic radii, ionic radii, important oxidation states, ionization energy, hardness, melting points, and density. 5. List at least five characteristics that the transition elements have in common with each other.6. Write the electronic configurations of the first row transition elements, noting the anomalies and the reason for them. 7. Define "lanthanide contraction" and predict its effect on the properties of the transition elements in period 6. 8. Predict the possible oxidation states of the transition metals and give the more important oxidation states of the first row transition metals. 9. Indicate the relative importance of higher and lower oxidation states as one moves horizontally or vertically among the transition metals. 10. Use the relative stabilities of oxidation states to determine which of 2 compounds will be more easily oxidized (or reduced) or which will be the better oxidizing agent (or reducing agent). 11. Give formulas and names to the more important oxides and hydroxides (and their anions) of the first row transition metals and compare their relative oxidizing abilities. 12. Compare the relative acidity of the oxides and of the hydroxides of each transition element that has more than one important oxide of hydroxide. 13. Explain the use of silver in the black and white photographic process. 14. Explain the physiological action of mercury. 15. Discuss the coinage metals and why they are called that. 16. Discuss the two oxidation states of mercury and the unique structure and bonding they produce. 17. List the iron, palladium, and platinum triads, and why Group VIII is structured that way. 18. List the platinum metals and some of their important properties. 19. Define or describe the following terms relating to metallurgy, giving examples where appropriate: a. Ore i. Blast furnaceb. Amalgam j. Cast ironc. Flotation process k. Pig irond. Gangue l. Steele. Slag m. Calcinationf. Flux n. Bessemer converterg. Roasting o. Open hearth furnaceh. Smelting p. Mond process20. Identify and describe the three main steps involved in extracting a metal from its ore, and give examples of each. 21. Define an alloy. 22. Name at least two important alloys and describe their composition and applications. 23. List at least two properties in which an alloy differs from its components. 24. Define and compare the terms paramagnetism, ferromagnetism and diamagnetism, and give examples of elements exhibiting each type. 25. Define the term domain and relate it to the degree of magnetism which a substance exhibits. 26. Define the following terms relating to coordination chemistry, giving examples where appropriate: a. Complex compound l. Enantiomersb. Coordinate covalent bond m. Racemicc. Ligand n. Inner orbital complexd. Coordination sphere o. Outer orbital complexe. Chelating group p. High spin complexf. Monodentate ligand q. Low spin complexg. Polydentate ligand r. Degenerateh. Coordination number s. Crystal Field Theoryi. Stereoisomerism t. Crystal field splittingj. Geometrical isomerism u. Valence Bond Theoryk. Optical isomerism v. Donor atom 27. Given the formula or structure of a transition metal complex, identify the ligands, chelating groups, coordination sphere, coordination number, and donor atom. 28. Name transition metal complexes (using the rules of nomenclature) given the formula, and vice versa. 29. Identify and draw isomers of some transition metal complexes, identifying cis, trans, and optical isomers, or given the structure, identify which isomer is present. 30. State what nonsuperimposable mirror images means and how this relates to coordination compounds and optically active coordination compounds. 31. Define polarized light and explain what happens to it when it is passed through a solution of each of a pair (or a mixture) of optical isomers.32. Use the valence bond theory to explain the nature of the bonding in coordination complexes. 33. For transition metal complexes, use the valence bond theory to explain: a. The nature of the coordinate covalent bond.b. Their electron configuration (before and after complexing).c. Their structure, geometry, and hybridization.d. Whether an inner or outer orbital complex will form (be more stable).e. The number of unpaired electrons that results.f. Their magnetic properties.g. Their color.h. The faults in the theory. 34. For transition metal complexes, use the crystal field theory (ligand field theory) to explain: a. The nature of the bond formed and compare it to the coordinate
covalent bondb. The effect of the ligands on the energy levels of the central
metal ion.c. The splitting and labeling of the energy levels above.d. The crystal field splitting, D, and its relationship to the
spectrochemical series of ligands.e. Their geometry or structure.f. The number of unpaired electrons that results, including
whether low spin or high spin complexes will result.g. Their magnetic properties.h. Their color.i. Their relative stabilities. Unit X: A. Nuclear Chemistry: 1. Define or describe the following terms, using examples where appropriate: a. Nuclide m. Acceleratorb. Isotope n. Natural radioactivityc. Alpha (a) particles o. Nuclear transformationd. Beta (b) particles p. Band of stabilitye. Gamma (g) rays q. Electron capturef. Parent/daughter isotopes r. Magic numbersg. Radioactive (decay) series s. Nuclear fissionh. Half-life t. Nuclear fusioni. Atomic mass u. Critical massj. Mass number v. Plasmak. Transmutation w. Chain reactionl. Nuclear force y. Induced fission2. Describe the composition of the nucleus. 3. Explain the factors influencing the change of an unstable nucleus to a more stable nucleus. 4. Describe natural radioactivity and the types of decay it produces. 5. Give the symbols and properties for the three basic emissions in natural radioactivity (a,b,g). 6. Complete and balance nuclear equations given all but one reactant or one product (using nuclear notation). 7. Explain the kinetics of radioactive decay using equations. 8. Apply the kinetics of radioactive decay to calculate half-lives, amount of sample left, original amount of sample, or elapsed time, given three of them. 9. Discuss the application of radioactive decay dealing with archaeological (carbon) dating. 10. Give reactions that tend to bring unstable nuclei into the band of stability. 11. Explain how "magic numbers" predict the stability of super-heavy elements. 12. Explain the principle of operation of: the Geiger Muller counter, the cyclotron, a nuclear reactor. 13. State and explain Einstein's equation relating energy to mass
(E = MC2) and relate it to nuclear fission and fusion. 14. Explain the relationship of nuclear fission to the atomic bomb and to nuclear energy. 15. Explain the relationship of nuclear fusion to the hydrogen bomb, to nuclear energy, and to the sun's energy. 16. Give examples of chemical applications of nuclear reactions. 17. Discuss all sources of energy in terms of safety, pollution and availability of fuel. 18. Discuss the basic principles in the operation of an atomic and hydrogen bomb. Unit X: B. Qualitative Analysis: 1. Know the cations in the groups we studied in the laboratory. 2. Know the reagents and ions responsible for the precipitation of each of the five groups. 3. Explain and interpret a flow chart. 4. Given an appropriate flow chart and some qualitative test results, determine which cations (or anions) might be present or absent from an unknown solution containing one or more ions from that flow chart. Be able to also do this in the laboratory. 5. Given a flow chart, explain how one would determine whether a particular ion on it were present or absent from an unknown solution containing one or more ions from that flow chart. 6. Explain and describe the chemical properties used in qualitative analysis as they relate to the appropriate objectives in the units on: "Electrochemistry", "Acids and Bases", "Ionic Equilibria", "Chemistry of the Representative Elements I and II", and "The oxidation-reduction, weak electrolytes, precipitation, solubilization, neutralization, amphoterism, hydrolysis, indicators, and coordination chemistry. Unit XI: Organic Chemistry: 1. Define the following terms, citing examples where appropriate: a. Organic chemistry l. Homologous seriesb. Aliphatic hydrocarbon m. Derivativec. Aromatic hydrocarbon m. Structural isomerd. Alkane o. Geometrical isomere. Alkene p. Optical isomerf. Alkyne q. Asymmetric carbon atomg. Alkyl group r. Functional grouph. Saturated s. Resonance hybridi. Unsaturated t. Resonance stabilization energyj. Cyclic compound u. Open structurek. Olefin v. Condensed structure 2. Describe what is meant by sp3, sp2, and sp hybridization of a carbon atom, and illustrate and name the geometry that results from these hybridizations. 3. Draw a representation of a sigma (s) and a pi (p) bond between carbon atoms. 4. Draw a valence bond representation of ethane, ethylene, and acetylene, labeling the bonds sigma or pi as appropriate. 5. Give four reasons for the fact that there are so many compounds of carbon. 6. Write the general molecular formula for any alkane or alkene. 7. Draw and name the structural isomers of the first ten alkanes, first ten alkenes, and the first ten alkynes. 8. Derive the correct names of any given compounds from the structures for the alkanes, alkenes, or alkynes, including cyclic hydrocarbons and substituent groups up to four carbons (and vice versa). 9. Given the formula for hydrocarbon, write the structures for the different isomers. 10. Distinguish among the boat and chair forms of cyclohexane. 11. State the common names of some of the simpler organic compounds. 12. Give uses for the first 10 alkanes. 13. Name and give the structure of benzene and its substituted derivatives, distinguishing between ortho, meta and para, where appropriate. 14. Sketch the resonance hybrids for benzene and explain how they relate to the real structure. 15. Identify and name the following compounds (and their functional groups) given the structure, and vice versa: a. Alkanes e. Ethers j. Carboxylic acids b. Alkenes f. Aldehydes k. Esters c. Alkynes f. Ketones l. Mercaptans d. Halides h. Amines m. Dissulfides e. Alcohols i. Amides n. Amino acids 16. Describe the synthesis of some simple alkanes, alkenes, alkyl halides, alcohols, esters, carboxylic acids, aldehydes and ketones. 17. Define, recognize and give examples of the following organic reactions: a. Substitution d. Oxidation b. Addition e. Reduction c. Elimination f. Esterification 18. Chemically and physically distinguish between: a. Primary, secondary and tertiary alcohols b. Aldehydes and ketones c. Alkanes and alkenes Course Laboratory Objectives: 1. Expand your understanding of the Course Objectives.
2. Demonstrate the ability to correctly and effectively manipulate chemicals and glassware by working alone.
4. Demonstrate the ability to correctly and effectively use laboratory balances.3. Demonstrate the ability to correctly and effectively collect and analyze data from an experiment by working alone.
5. Demonstrate the ability to correctly and accurately do quantitative analysis such as titrations, pipetting and preparation of solutions by working alone.
6. Demonstrate the ability to correctly and effectively collect and treat data on the computer.
7. Demonstrate the ability to correctly and effectively use instruments like Spectrophotometers, voltmeters and pH meters.
8. Utilize critical thinking and quantitative reasoning skills in observing, organizing and analyzing data, synthesizing information, interpreting results, and communicating the results of the analyses and laboratory investigations.
9. Perform chemical experimentation in a safe and scientific manner, using proper scientific and laboratory safety procedures.
10. Students must show work, thought process and/or justification for answers when
necessary on laboratory reports. They should also be clear and legible.